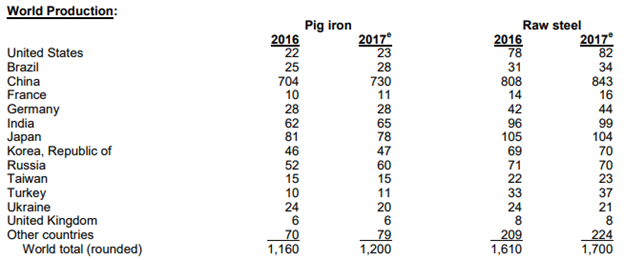
Before we can get into the history of steel and discuss its importance to our daily lives, we should take a look at global production of iron and steel, as there has been a series of very interesting developments recently that have the potential to greatly impact the steel industry, but also the global economy. According to the United States Geological Survey (USGS), the largest producer of steel in the world is by far and away China, with an estimated 843 million tons in 2017. The next closest to China is Japan with 104 million tons in 2017 followed by India and the United States with 99 and 82 million tons produced respectively.
Note: Millions of tons; Source: USGS
China’s steel mills produce around one-half of the world’s steel. The country exports about 100 million tons worldwide every year and therefore has great influence on global steel markets. A number of trade petitions have been made in recent years from North America and Europe addressing China’s excess capacity. In response, China stated its intent to reduce steel output, pledging to remove some 150 million tons per year of steel production capacity by 2020. This would reduce capacity to about 700 million tons.
After the American Society of Civil Engineers gave the United States infrastructure a D+ grade in 2017 and called for the use of American steel in infrastructure projects, the Federal Government began an investigation to determine if steel imports are a risk to national security. The steel industry in the U.S. has asserted that it is indeed a threat to national security as the practices of governments of other steel-producing countries distort global markets. However, there are others that believe that the investigation and any subsequent measures to deter such practices from other countries will be detrimental to steel consuming industries in the United States that depend on a reliable and economical supply of steel imports.
Nonetheless, on 1 March, President Donald Trump announced he would impose massive tariffs on all imports of steel and aluminium. After many countries prevailed upon the U.S. with their objections, the Trump administration moved forward with tariffs, exempting the EU, Canada, Mexico, Argentina, Australia, Brazil, and South Korea. However, this will do little to calm those that fear that a trade war between the U.S. and China is on the horizon.
An open trade war among major economic players would disrupt global commerce, which has been the main driver of global growth in recent years. For now, China, Trump’s main target because of what he calls China’s “unfair” trade surplus with the U.S., has reacted cautiously to the U.S. tariffs on aluminum and steel, as they would have a negligible effect on its economy. However, China has a lot to lose should an open trade war with the U.S. materialize given the massive trade surplus that the country holds with the U.S. and the importance of the export sector to the economy.
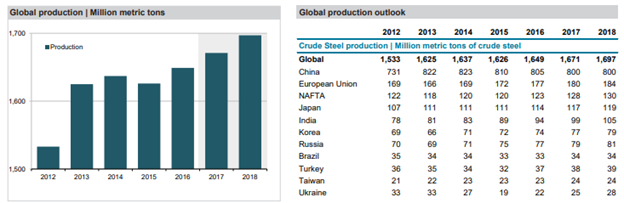
It will be interesting to see how this all plays out and how this affects steel prices and steel production in the near future. According to the Focus Economics Consensus Forecast Commodities, “growing uncertainty in the market and the ongoing supply glut will keep prices constrained despite a moderate pick-up in demand.” European steel prices are forecast to average USD 506 per metric ton in Q4 2018 and USD 468 per metric ton in Q4 2019 while U.S. steel prices are projected to average USD 591 per metric ton in Q4 2018 and decrease to USD 574 per metric ton in Q4 2019.
You can download a free sample of our Consensus Forecast report for Commodities here.
Source: FocusEconomics Consensus Forecast Commodities
Steel History
The story of steel began thousands of years ago with the human discovery of iron during the 4th Century BC. There is evidence that in Egypt beads of iron were taken from meteors. This meteoric iron was a highly prized material due to its association with the heavens. The only naturally occurring iron on the face of the Earth, even to this day, comes from meteors, as iron reacts with oxygen to create iron ore. Since there is no oxygen in space, the only way our ancestors were able to salvage iron was from meteors. This was of course until we finally learned to extract iron from its ore, which marked the beginning of the Iron Age.
The Iron Age began when humans were first capable of producing iron from iron ore. This began at different times in different places around the world, however, it’s generally thought to have begun some time during the 2nd Century BC as the Bronze Age came to a close. The advantage of iron over bronze was that bronze is an alloy of tin and copper, which were metals not easily collected, as they were not often located near one another. The alloy was therefore more onerous to produce than iron, which could be made from one abundant material, iron ore. It could be argued that the Iron Age actually continued up until around 150 years ago when steel first began to be mass produced.
Although the Iron Age is named after iron, steel was produced dating all the way back to the beginnings of the Iron Age. That is because steel is essentially iron with a specific percentage of carbon. The Roman Empire made weapons from Noric Steel. This steel came from the Celtic kingdom Noricum, located in modern day Austria. Demascus steel, or Wootz Steel, was produced in south India, as early as the 6th Century BC, while in China, steel was produced as early as the 4th Century BC.
Some of the best materials were made with steel, however, the main issue with producing steel of course was getting the carbon content just right. Any iron with a carbon content above 2% is considered cast iron. This resulted in a harder and less ductile material. Ductility is the degree to which metals can be stretched. Although cast iron was very hard it was also very brittle, making it less than ideal for shaping and working. Nonetheless, it was cheap to manufacture, which made production attractive, however, it was not suitable for many things, especially structural use. For example, during the 19th Century, many bridges were constructed with cast iron and collapsed due to iron’s brittle nature.
After some time, metallurgists came to realize that the problem of cast iron’s brittleness had to do with the higher carbon content. Thus, they sought a way to make more workable iron with less carbon. Wrought Iron was therefore adopted as the main iron to be used for structural purposes. Wrought iron contains less than 0.8% carbon, which made it much more useful for the construction of bridges, for example, as it was more ductile and therefore able to bend and sway without breaking. However, because of its low carbon content, wrought iron was much softer than cast iron, which is where steel comes into the picture.
Steel is made with just the right amount of carbon, 2%. It is the happy medium between strong cast iron and ductile wrought iron. However, to understand the history of steel making, it’s important to understand the production methods of iron dating back to the beginnings of the Iron Age.
Steel Production History
Because iron readily reacts with oxygen to produce iron oxide ores, or rust, its prevention is a constant battle. The Eiffel tower, for example, was built with iron and is painted every seven years with 60 tons of paint, to keep it from rusting. Because Iron does not exist on Earth in a naturally occurring and useable way due to its reactions with oxygen, the first step in producing iron from iron ore, is to remove the oxygen.
Iron was originally produced in what are called bloomeries, which were primitive furnaces in which charcoal was burned to produce carbon monoxide. The carbon monoxide would react with the oxygen in the iron to remove it. The fire would burn at a temperature below the melting point of iron, but above that of the ore’s impurities. The molten pool of impurities, known as slag, could be drained away, leaving just the iron. Any leftover impurities would be worked and hammered away.
These bloomeries produced very small quantities of iron, especially before the development of the water wheel to power them. Despite the small quantities they produced, bloomeries revolutionized everyday life. As mentioned previously, prior to the Iron Age, bronze was the preeminent metal, however, it was far more difficult to produce due to the fact that it was an alloy of tin and copper. When humans learned to produce iron from iron ore, this changed everything, as just about anyone could create tools and weapons from iron ore, as it is far more common than copper or tin.
With society becoming more stratified and trade increasing, more efficient processes to produce iron were needed, like the blast furnace. The blast furnace was originally used as far back as 100 AD in China, however, it made its way west slowly and was introduced in Europe during the 15th Century.
The blast furnace was able to increase the production of iron dramatically. Preheated air at about 1000 degrees Celsius is blasted into the furnace from nozzles at its base. Contrasting with the bloomery, the iron in blast furnaces is heated to above its melting point, along with flux materials. Flux is the purifying agent that purges the metal from the chemical impurities and allows the iron to be extracted more easily. Flux in this case is limestone and coke. Coke is a refined bituminous coal, also known as coking coal, with very few impurities. Similar to the use of charcoal in bloomeries, coke produces carbon monoxide when burned which in turn reacts with the oxygen to remove it. The limestone reacts with the heat to create a slag of impurities that floats at the top of the blast furnace, which can then be drained away, leaving the heavier molten iron below.
Blast furnaces allowed vast quantities of iron to be produced relative to bloomeries, however, one drawback was that at high temperatures, iron reacts with carbon to absorb it, meaning that the iron produced was cast iron. Additional steps were needed to decrease the carbon content, which is called fining. The cast iron would be heated back up, oxidizing the iron, then the material would be beaten with a hammer to knock the oxidized carbon out, which was repeated until the iron was considered wrought iron.
Unfortunately, this process was fuel and labor intensive and therefore not suitable for industrial use. With the industrial revolution and the growth of rail roads, great pressure was thrust upon the iron industry to find a solution to create faster more efficient production processes.
Along with the inefficient production process associated with wrought iron was that it was too soft for things such as train tracks. Train tracks would be needed to be replaced every 6-8 weeks in some cases due to the rapid wear and tear on the soft metal. Steel therefore became the metal of choice for rail roads, as it is stronger and able to resist wear.
However, steel was still unproven as a structural metal and production was still slow and costly. With the Industrial Revolution well under way, the increasing globalization and industrialization of the world necessitated a means of mass production of steel. This is where Sir Henry Bessemer came in with the Bessemer Process.
Henry Bessemer designed a converter in which molten iron was poured from the blast furnace. Hot air was passed through the bottom, oxygen from the air oxidized the impurities in the iron and the carbon reacted to form carbon monoxide and was expelled as a gas.
The Bessemer Process was very fast and inexpensive, unfortunately however, early on the process was a victim of its own efficiency, as it expelled too much carbon and left too much oxygen in the iron. To solve the problem, a compound was added to the process, called Spiegeleisen, which was an alloy of iron and manganese. If added in the right quantities, the manganese would react with the oxygen to remove it and the iron increased the carbon as needed.
Yet, another issue arose. Phosphorus needed to be removed from the process, as high concentrations of the chemical element make steel brittle. Initially the Bessemer Converter could only be used with iron obtained with low phosphorus concentrations, which was scarce and therefore expensive. It wasn’t until 1876 when a Welshman named Sidney Gilchrist Thomas solved the problem by adding a chemically basic material to the Bessemer Process, limestone, which would draw the phosphorus into the slag. Finally the process was perfected and steel production skyrocketed as a result and so did railway construction.
By the 1860’s a new method of mass production was created by German engineer Karl Wilhelm Siemens called the Siemens-Martin Process. This process produced steel from iron ingots, also known as pig iron, in large shallow open-hearth furnaces. The process relied on heated brick chambers below the furnace to maintain temperatures high enough to burn off excess carbon and other impurities.
The advantage of this method was that it produced steel in even greater quantities than the Bessemer Process; 50-100 metric tons could be produced from a single furnace. The process was fairly slow in comparison to the Bessemer Process, however, this allowed for better quality assessment to make steel to exact specifications. Despite the slower process, the advantages proved to outweigh the disadvantages and by 1900 the open-hearth furnace had largely replaced the Bessemer Process.
How is steel produced today?
Basic Oxygen Process
The basic oxygen process (BOP) involves blowing pure oxygen into a blast furnace with molten iron and scrap. This initiates a series of exothermic reactions which remove impurities such as carbon, silicon, manganese and phosphorous. Although the efficiencies of pure oxygen steel making were originally noted by Henry Bessemer, the process could not be brought to mass production of steel until the 20th century when large quantities of high-purity oxygen became available. Some of the commercial advantages to the BOP include high production rates, less labor, and low nitrogen steel. These efficiencies made the open-hearth process largely uncompetitive during the last century and the BOP eventually replaced all open-hearth steel making processes. The last one in the United States closed in 1992 while in China the last one closed in 2001. Around 66%-75% of the world’s steel is today produced by the BOP method.
Electric Air Furnaces
The leaps and bounds made in electrical engineering toward the end of the 19th century and into the 20th century presented the steel industry with new opportunities in steel production. The Electric Arc Furnace (EAF), which was first developed just after the turn of the 20th century, is still used to this day. Although electric arc was experimented with to melt iron as early as 1810, Frenchman Paul Héroult was the first to employ the idea to steel production with a commercial plant in the United States in 1907. The EAF was designed to pass an electric current through a charged material, which heats the iron up to temperatures of 1800 degrees Celcius.
Although originally used for specialty steels, such as steel made for springs and machinery, EAF use for long steel manufacturing, such as structural steel, rod and bar, and wire, has grown due to a number of advantages. Low investment costs are associated with EAFs since they use what is called a minimill in contrast to the more expensive integrated mills. During World War II, the low capital investment associated with the EAF allowed war ravaged Europe to compete with some of the bigger U.S. steel making firms such as U.S. Steel and Bethlehem Steel. Nucor is currently one of the largest steel making firms in the U.S. and employs exclusively minimills and EAFs.
EAFs have various other advantages. EAFs are able start and stop production as needed, something blast furnaces cannot generally do. This allows EAFs to vary production according to demand. EAFs also allow for the production of steel from 100% scrap metal. This greatly reduces the energy required to produce the steel compared to steel making operations from iron ore. Although EAFs usually create steel from scrap metal, molten iron from a blast furnace can also be used. EAFs are growing rapidly and are currently responsible for about a quarter to a third of the world’s steel production.
Steel Uses
Steel is so vital in our daily lives, we often consider it a measure of the economic success of a country. If there is high production of steel, that means there is a high demand for steel, meaning the country is building infrastructure and growing economically. But what exactly is steel used for? Let’s take a look.
Construction
Steel is mostly used in construction. In its various forms and alloys, steel is designed to meet the specific requirements of unique projects. This allows steel to be incorporated in infrastructure projects in all environments. During the 20th century with the advent of more efficient steel production processes, the railroads expanded greatly as did the advent of high-rise buildings.
Steel is also used in the construction of just about anything you can think of, such as stadiums, bridges, piers, harbors, roofing, tunnels, etc.
Transport
Steel is heavily used in the automotive industry. Steel accounts for about 50% of the weight of the average automobile. These steels are engineering steels, which are designed to have certain specifications of elasticity, ductility, and resistance to corrosion. Steel is also used to make trucks, transmissions, trains and railways, ships, anchors, and aircrafts.
Energy
The energy sector relies greatly on steel for its infrastructure. Steel can also be used for resource extraction, such as in offshore oil rigs and pipelines, earth-moving equipment, cranes, and forklifts. In addition, the following energy projects also rely on steel, such as electricity power turbine components, wind turbines, electromagnets, transformer cores and electromagnetic shields.
Packaging
Steel is used in the packaging industry, as it protects the contents of the package from the elements such as air, water, and light exposure. The majority of steel packaging is used in the food and beverage industry as well as for aerosols and closures, for example, bottle caps.
Appliances
About 75% of the weight of the typical household appliance is made of steel. You can find steel in just about any household appliance such as refrigerators, washing machines, ovens, sinks, cutlery, microwaves and the list goes on.
If you have any other questions related to steel or would like to suggest another commodity for us to cover in our next explainer post, send us an email at blog@focus-economics.com. Also, don’t forget to download one of our sample reports by clicking on the button below.
Read some of our other Commodities Explainer posts:
What is the difference between Brent and WTI crude oil?
Gold: A History of Obsession – Part 1
Gold: The most precious of metals – Part 2
Gold: The most precious of metals – Part 3
Iron ore: A most underappreciated commodity
Copper: The first metal mastered by man
Coal: The story of the world’s most abundant fossil fuel
Sample Report
5-year economic forecasts on 30+ economic indicators for 127 countries & 33 commodities.